Company Profile
| Company name | Phicell Corporation |
| Establishment | November 15, 2017 |
| CEO | Etsuko Sonoi |
| Business content | Storage and management services for bio samples, specimens, cells, and investigational drugs to support research and development in pharmaceuticals, medical devices, and regenerative medicine products. |
| Location | Head office:1-5-2 Minatojima-minamimachi, Chuo-Ku, Kobe, Hyogo Tokyo branch:1-31-18 Takabanobaba, Shinjuku-Ku, Tokyo Osaka branch:6-2-6 Fukushima, Fukushima-Ku, Osaka, Osaka |
| Affiliate | Saroute Co., Ltd.(https://www.saroute.co.jp/) |
Our History
| Nov 15, 17 | Phicell Corporation was established in Takadanobaba as a 100% subsidiary of Saroute Co., Ltd. At the same time, Kobe Laboratory was opened in Kobe, Hyogo. |
| Nov 20, 17 | Phicell was selected as a specimen storage management joint venture by the joint venture selection committee utilizing Translational Research Center for Medical Innovation. |
| Dec 16, 17 | Commencement of the specimen storage management business. |
| Oct 1, 18 | Commencement of the specimen storage management business at a GMP-compliant level. Commencement of contract storage management services for regenerative medical products. |
| Oct 1, 19 | Began contract work for material low temperature durability tests (demonstrations). |
| Nov 15, 19 | Launch of Japan’s first cloud 3D specimen storage management web app “Cellaph”. * |
| Dec 20, 21 | Kobe office moved from the 7th to the 2nd floor of the Kobe KIMEC Center Building. |
| Aug 1, 22 | Establishment of a new wet laboratory and storage room. |
| Jun 14, 23 | Head office moved from Takadanobaba, Shinjuku-Ku, Tokyo to Chuo-Ku, Kobe. |
| Sep 29,23 | Establishment of the new Osaka branch office at Fukushima, Fukushima-Ku, Osaka. |
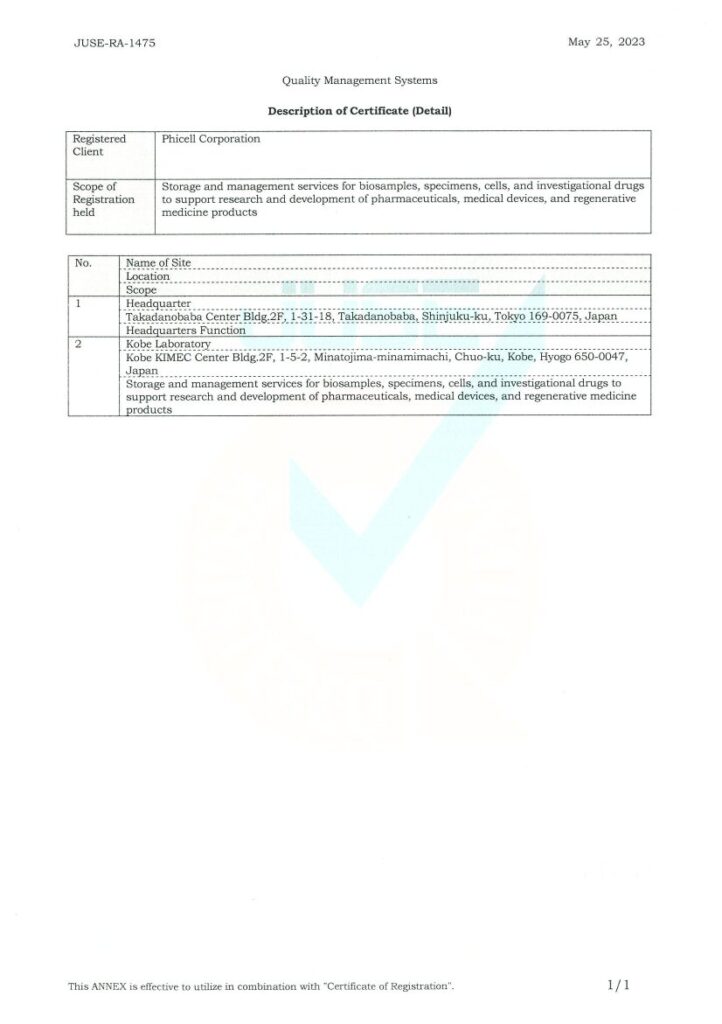
Certification
| Registration type |  Quality management system Quality management system |
| Applicable standards | JIS Q 9001:2015(ISO 9001:2015) |
| Registration range | Storage and management services for bio samples, specimens, cells, and investigational drugs to support research and development in pharmaceuticals, medical devices, and regenerative medicine products. |